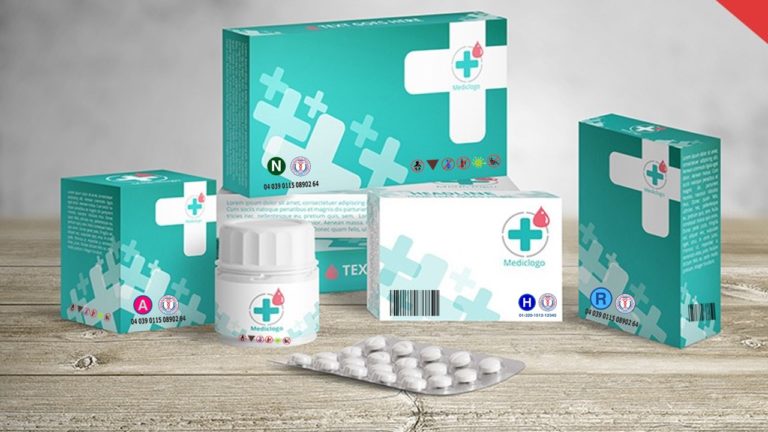
REGULATORY INFORMATION
Public and Patients in UPCOS
The availability of safe, reliable and effective medicines of good quality give patients confidence and trust. When patients are prescribed medicines for prevention or cure of disease they have a right be informed that the medicines they take are safe and reliable. It is ultimately in patients’ interest that manufacturers and Drug Regulatory Authorities, with the responsibility for the safety, efficacy and quality of the drugs marketed for public consumption do their best to sustain the patient and drug safety.
The patient is, however, continues to remain vulnerable. The facilitated the uncontrolled internet sale of medicines (including herbal and traditional medicines) across borders, for instance, poses serious problems that involve alarming safety, efficacy and quality issues related to prescription drugs, unregistered medicines, highly controlled substances and traditional and herbal medicines.
The frailty and vulnerability in interstate/international pharma trade regulations bring more complications in drug safety; a drug, approved and licensed in a country, may not be approved or licensed in another one whether it is a neighboring or distant country. This is the situation today even in the EU countries simply because there are currently no conventional rules or regulations to eliminate such incompatibilities.
All of that urgently brings the vital necessity of a publicly open, unbiased and flawlessly homogeneous universal pharma coding system: That is uniquely UPCOS.
Reliable public application and availability of sound, trustworthy, safe and secure medicines at the highest levels is now possible with the unique UPCOS System which provides patients for the first time in the world with the information that the medicines they take are secure, safe and reliable letting them also know what kinds and groups of medicines they are and where they come from; are they coming from a secure and trustworthy place or from an obscure unknown source.

UPCOS Safety
The aggressive marketing of medical products in general as well as alternative medicine and biomedical products and the virulent medical products marketing through the Internet, in particular, with important consequences on public health and safety today brings the necessity for the creation of a system for global measures of drug safety concerns.
So far, the public awareness of drug safety across the world has not been sufficient enough to prevent patients from the problems related to drug safety and efficiency and the problems of the pharma industry’s regulatory and commercial matters as well as the problems of provision.
While embracing the patient, the best form of safety, must also involve prescribers, manufacturers, drug authorities and the public as a whole. The pharmaceutical industry has prime responsibility for the safety of medicines giving the leading role to manufacturers in monitoring the safety of medicines from the start of drug development and thereafter throughout the lifetime of the drug.
That would not work without a wholesomely effective public pharma-coding system that is capable of gathering pharmaceutical industry, drug authorities, healthcare professionals and the public on a collaborative and communicative platform within an international harmony. This is what the public UPCOS System simply and practically brings to the world.


The WAMS Approval in UPCOS
As its creator, holder and administrator, the World Academy of Medical Sciences (WAMS) puts the UPCOS System in use within a unique collaborative network of the public, pharmaceutical industry, health professionals, governments, public health administration, regulatory authorities, media, and academia.
It assigns its logo to the UPCOS Coding system as the coding’s essential element which is displayed on products’ packages, bottles, boxes, tubes and inserts as the seal of approval, sanction, and endorsement of the products’ granted UPCOS codes.
By that, the academy takes every responsibility for securing the UPCOS System’s virtue and integrity in its official and public usage both technically and practically, and for dealing legally with all issues, matters and cases that may be related to or caused from abuse, misuse, mismanagement or pervertion of the system.