UPCOS IN BRIEF
HISTORY
Life on our beautiful planet today is dominated by trade whose effects are amply lived by us in our daily life.
Those effects very often influence or even change directions of trade flows and sometimes with some unfavorable consequences.
We see such consequences also on the healthcare industry’s regnant trade side.
Well, since there is a harmonious series of systems that keep healthcare and trade mutually hand in hand, we shouldn’t really complain. But only up to the point that the systems get misused.
In today’s World we live in, we medical professionals, along with our colleagues in the global pharma industry, dedicate ourselves to our patients and to the people at large across the World.
We virtually live with our patients the same life together sharing the joint elements of living and well being facing challenges and conforming together to the values of human life we cherish.
With them, we, at the same time, jointly encounter the problems of healthcare systems and the problems on the pharma front and, in particular, the problems where the results affect not only patients and health professionals, but also health authorities and governments.
In 2017, the amount of money the world spent on prescription drugs was around $1.3 trillion.
The money spent on non-prescription (over-the-Counter [O/C]) drugs, alternative medicine drugs and substitutes, cosmetic and hygienic products and biotech products altogether in the same year is estimated to be over $1.6 trillion. This is a Total of almost 3 trillion.
About a fifth of that amount, around $600 billion, is the money lost because of the black market sales, undocumented and counterfeit sales, fraud, corruptions, misuse of trade regulations and malpractice and misconduct, etc.
Countless efforts over the years and decades, globally, have been being made to solve those problems of the pharma industry from which the public, as well as the industry itself, continue to suffer facing the never-ending and ever-growing disorders.






FDA ADVISES THE PUBLIC
“FDA takes all reports of counterfeit drugs seriously and, in order to combat counterfeit medicines, is working with other agencies and the private sector to help protect the nation’s drug supply from the threat of counterfeits.”
…. And counterfeit trade and health frauds still go on and more than ever.
And it gives standard petty tips to the consumer on how to identify a counterfeit medicine as below:
– Check the shape and color of the medicine (tablet, etc)
– Check the shape and color of the box or bottle of the medicine
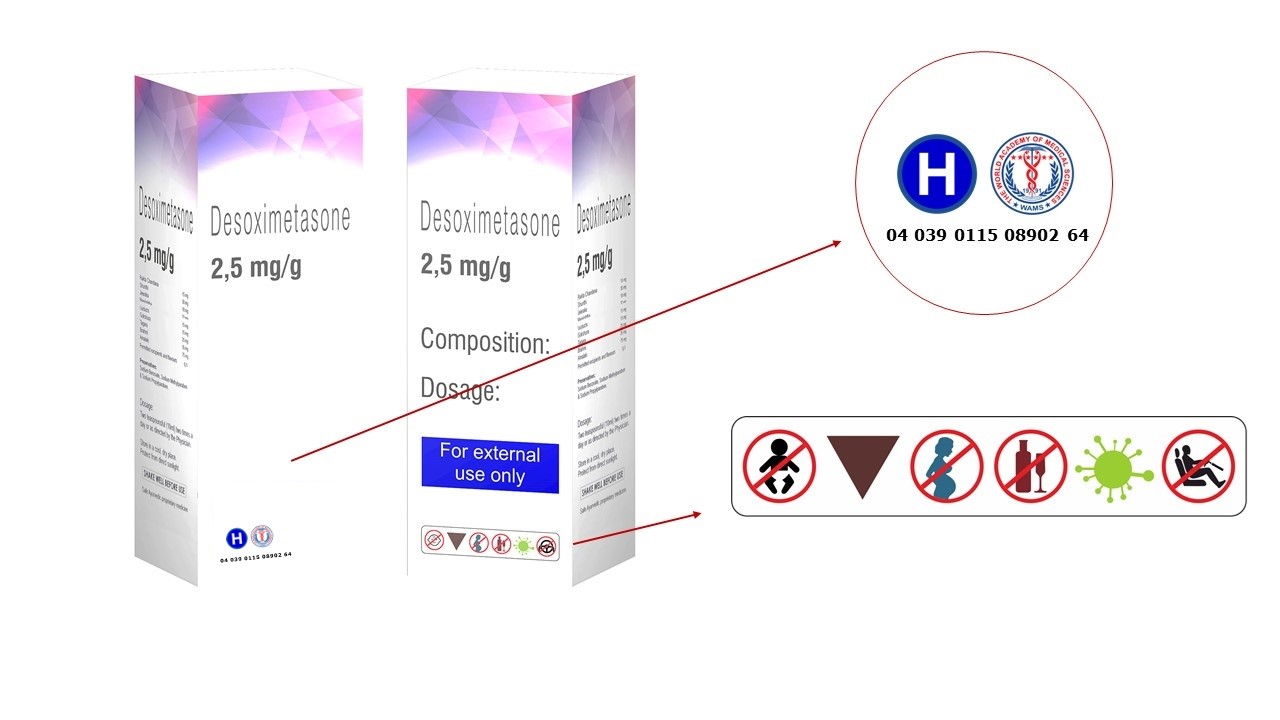
– Check the serial number, drug code number and lot number, etc.
Counterfeiters do their work professionally and make every feature identical to the original.
…. And counterfeiting goes on.
FDA gives more standard public tips on how to identify rogue online pharmacies:
Beware of online pharmacies that:
– Allow you to buy prescription medicine without a valid prescription from your health care provider.
– Do not have a U.S. state-licensed pharmacist available to answer your questions.
– Offer very low prices that seem too good to be true.
– Send spam or unsolicited email offering cheap medicine.
– Are located outside of the United States or ship worldwide.
These pharmacies often sell medicines that can be dangerous because they may:
– Have too much or too little of the active ingredient you need to treat your disease or condition.
– Not contain the right active ingredient.
– Contain the wrong or other harmful ingredients.
The FDA tips on how to know if an online pharmacy is safe:
There are ways you can identify a safe online pharmacy. They:
– Require a valid prescription from a doctor or another licensed health care professional.
– Are licensed by your state board of pharmacy, or equivalent state agency. (to verify the licensing status of a pharmacy check your state board of pharmacy.)
– Have a U.S. state-licensed pharmacist available to answer your questions.
– Are in the United States, and provide a street address.

While useful and necessary, these tips and advises are not quite sufficient or good enough to provide the public with fully embracing safety, security, reliability or trust.
The UPCOS, the first ever and only public Universal Pharma-Coding System provides an official international platform of public pharmaceutical coding and monitoring from which, the public, states, medical professionals, medical, healthcare and pharma authorities as well as the Pharma industry itself will benefit invaluably. Via its World Charter, set in accordance with governments and pharmaceutical industry as well as food and drug administrations, healthcare professionals and the public on a collaborative and communicative platform within an international concordance. The UPCOS System will fully be implemented in 2020 globally.
In the practice and industry of the world’s chemical and pharmaceutical fields as well as our own practice of medical arts and sciences, both professionally and academically, we every now and then run into some various sorts of pharmacological and chemical codes and identifiers of drugs, medications and chemical substances which the public have no idea what they are about. As a physician, I have never needed to use any of them in my life. Currently in use, those useful codes and identifiers are all technical rather than practical paradigmatic platforms. Among them are standardized chemical nomenclatures (IUPAC), numerical identifiers (CAS), pharmacological data and target nomenclatures of chemical molecules (PubChem), databases of information on drugs and drug targets (Drugbank), databases for chemicals (ChemSpider), alphanumeric identifiers with substance registration systems (UNII), chemical databases of bioactive molecules (ChEMBL), systems dealing with the registration, evaluation, authorization and restriction of chemicals (ECHA) and classifications of active ingredients of drugs (ATC), etc.
And yet, despite the vital need, there has not been a comprehensively available, simple universal public pharma coding system which can provide primarily the public, together with the professions and industries of medicine, pharma and chemistry as well as related governmental bodies, institutions and agencies, with overtly available information and guidance which brings safety, security, reliability and trust to all the medical, pharmaceutical and chemical products that directly relate to the public and directly involve the professionals and components of the medical, pharmaceutical and chemical industries.
This now becomes the honorable and praiseworthy duty and function of the unique UPCOS System. In some countries there are national drug codes giving code numbers to human drugs (e.g. NDC in the United States) Those numbers are present on medication packages and inserts. Such national coding systems do not practically solve the national and international public medical problems of the pharma industry as those problems are not limited to national domains or vicinity and they are as international and intercontinental as the legal universal pharma trade flow itself.
In the pharma industry and medicine, illegal, undocumented and counterfeit sales, fraud, corruptions, misuse of trade regulations and malpractice and misconduct, etc., are well known, a part of daily life in developing countries. Other commonly seen problems involving irrational drug use, poly-pharmacy and interactions, increasing the use of traditional and herbal medicines with other medicines, illegal online sale of medicines and drugs of abuse, increasing self-medication practices, substandard medicines, medication errors and lack of efficacy are the ones we see not only in the developing countries but also almost in all the western countries primarily including Europe and the USA.
Today, distressingly, however, we see those things, in some extent, in western countries too.
In a European country, I myself witnessed, some while ago, such a bad example: A limited-income patient of mine contacted me and told me that his pharmacist would not provide him with his prescribed vital medicine because no supplies were available at the moment. He however suggested him that the medication was available in the black market with a little higher price and he could give him a particular address where he could purchase it. He got the address but he did not go there and rather came to me. I was as much shocked and outraged as the patient. I got both the name of the Pharmacy and the address of the black marketeer from him and visited the both places thinking that the two were collaborators working together. The pharmacist told me that the decent market in town had been desperate for the unavailability of that important medicine and it was only his pure attempt to help the patient by redirecting him to the black marketeer whom he neither know, nor had any link with. I believed him and visited the black marketeer who was, in his explanation, as fair and open as the pharmacist. He said that the whole thing, nationwide, the result of some wrong government policies and it was happening to many other drugs. I told him I would continue to listen to him only if he would let me see a sample of the drug. He did. It was an unlicensed but license-pending generic drug. He said it was as good as the original one. I believed him and assured him that I would not report him to the authorities for the sake of patients who would suffer badly without that drug.
Anyhow, this was a typical example of only one of the multi-fold problems we currently experience across the World.
In the west, a black market player like this one can get away like this just because almost all of such unlicensed generic drugs is at least not counterfeit in Europe whereas in developing countries this is usually not the case; the drug made of starch or other filling materials with very little or no active ingredient and patients get more sufferance than cure.
Having been seeing this whole dramatic picture across the world for a long period of time, we see the necessity, importance and crucial impact of the UPCOS System which we have been being developing for the past seven years.